“Due to the fact 2014, Europe has also started next the USP approach of publishing particular person formulation monographs containing dissolution approaches and acceptance requirements,” adds Eckert.
Straightforward priming heater/circulator sits behind h2o tub to avoid wasting beneficial bench Room with small vibration
Store solutions from little company makes bought in Amazon’s store. Explore more about the tiny organizations partnering with Amazon and Amazon’s dedication to empowering them. Find out more
In addition, Clay emphasizes that as a result of a growing variety of badly soluble molecules coming into the development pipeline, the volume of elements falling right into a DCS course II or IV also are mounting.
The biphasic mini-tablets were formulated productively for much better control of drug release causes higher affected person compliance. The use of soluplus as a precipitation inhibitor is explored inside the oral sound dosage kind for the badly aqueous drug.
You should keep up The great do the job. Also, the complex providers employees and product sales rep are quite helpful and professional. Distek is the number one selection when considering our lab's dissolution apparatus."
The hole is equidistant from the center with the plate which is equally spaced from one another, that may be connected towards the lower facet of the lower plate is actually a piece of woven gauze created from stainless steel wire (10 mesh display screen size). These are generally 635 mm in diameter and also have a nominal mesh aperture of 2.00 mm.
The sustained launch mini-tablet was formulated utilizing Precirol ATO 5 and ethyl cellulose. Two-dimensional and a few-dimensional plots had been exposed the significant result of the level of Precirol ATO 5 and ethyl cellulose. The overlay plot locates the optimized region. The in-vitro drug release review exposed the desired drug launch of the ultimate mixed formulation. The in-vivo plasma concentration-time confirms the drug launch up to 12h.
“They may also be valuable in the development of generic products and solutions to check eroding and non-eroding matrices.”
Company and help from Distek is unparalleled while in the business. With above 45 several years of practical experience, Distek is properly-competent to satisfy the distinctive worries of the laboratory. Simply click the website link under to request assistance.
when basket-type apparatus is accustomed to allow the pill or capsule to sink to the bottom from the vessel previous on the rotation of the paddle.
This perform describes a microfluidic drug dissolution testing strategy which was created utilizing a business quartz crystal microbalance (QCM) resonator coupled with an axial microfluidic stream cell. Dissolution testing is employed to obtain temporal dissolution profiles of get more info prescription drugs, which provide information on the bioavailability or the drug’s capability to be fully dissolved and then absorbed and used by the human body. Feasibility in the QCM dissolution testing technique was shown utilizing a sample drug procedure of skinny movies of benzoic acid dissolved in water, capturing the drug dissolution profile beneath different microflow ailments.
The DT apparatus decides whether tablets or capsules disintegrate in just a suggested time the moment put inside a liquid medium. Table of Contents
In search of dissolution test apparatus that will Obtain your products and solutions to current market a lot quicker? Easily transition from R&D get more info to QC environments and realize dependable, reputable success for nanoparticle dissolution testing in an automated system whilst ensuring cGMP compliance.
 Charlie Korsmo Then & Now!
Charlie Korsmo Then & Now! Jennifer Love Hewitt Then & Now!
Jennifer Love Hewitt Then & Now!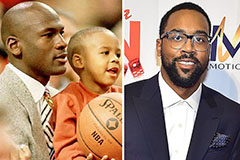 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!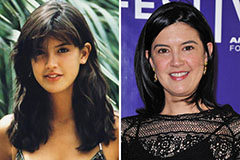 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Sarah Michelle Gellar Then & Now!
Sarah Michelle Gellar Then & Now!